Accurate chemical concentration measurement is the backbone of water treatment, agriculture, pharmaceuticals, manufacturing, and environmental monitoring. Yet, even experienced professionals make ppm measurement mistakes that compromise data reliability and decision-making.
A small error in ppm calculation can lead to:
- Incorrect chemical dosing
- Regulatory non-compliance
- Product quality failures
- Health and safety risks
- Financial losses
From misreading instruments to using the wrong ppm formula, these mistakes affect beginners and professionals alike.
This practical troubleshooting guide identifies the top 10 ppm measurement mistakes, explains their root causes, and provides clear solutions—helping you achieve consistent, defensible, and high-precision results.
What Is PPM and Why Accuracy Matters
Understanding Parts Per Million
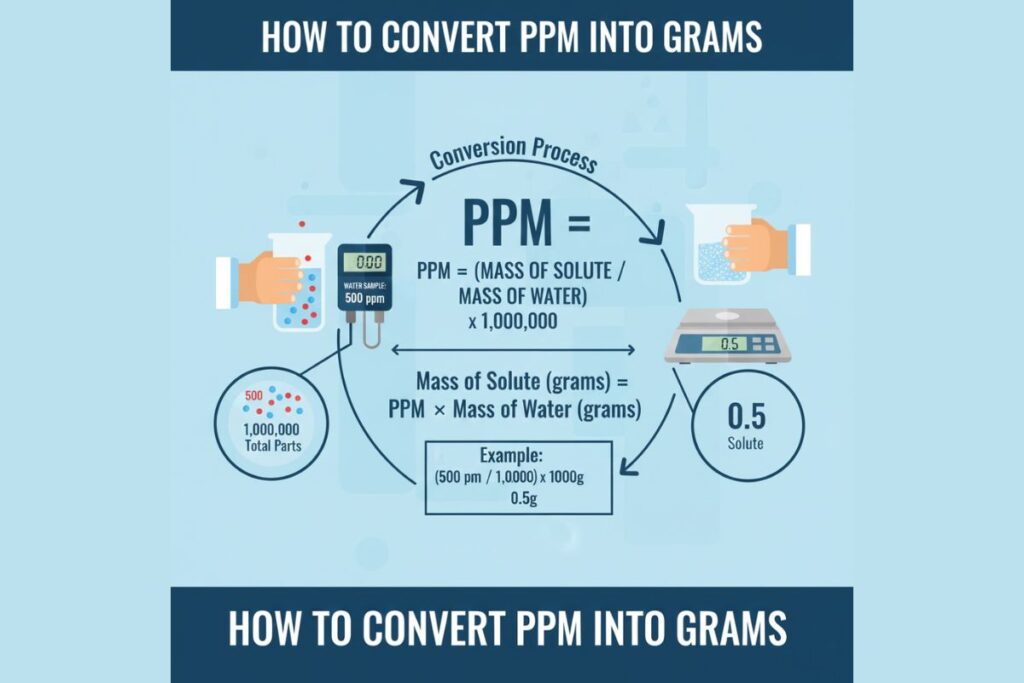
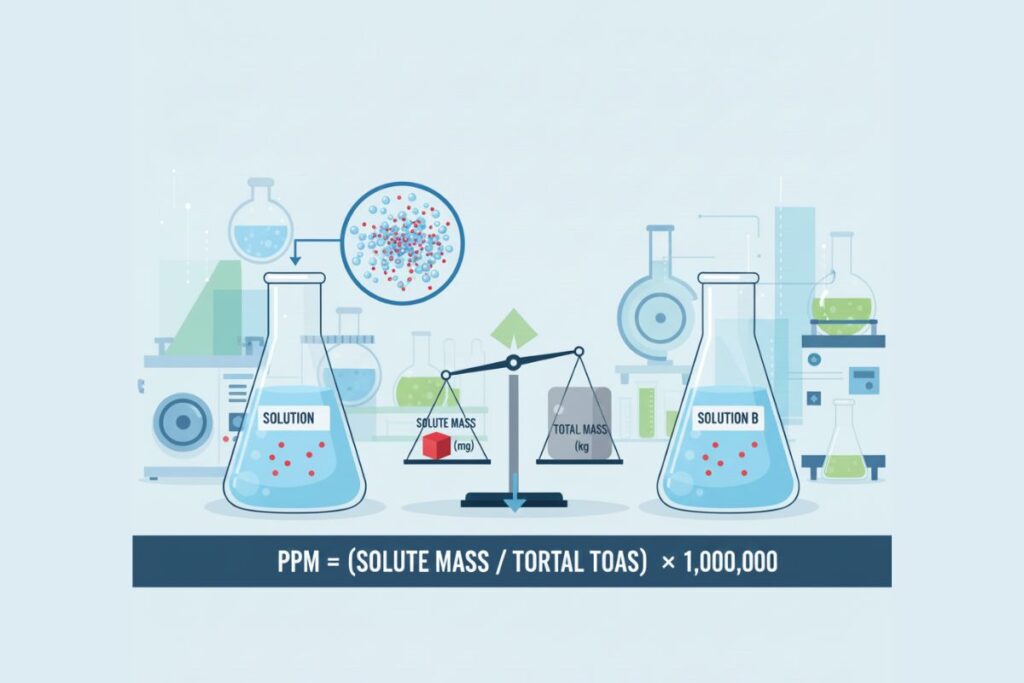
Parts per million (ppm) expresses the amount of a substance per one million parts of a solution or material.
In water systems:
1 ppm ≈ 1 mg/L
This equivalence simplifies regulatory reporting and laboratory analysis.
Importance of Precise PPM Measurement
Accurate ppm values support:
✔ Safe drinking water
✔ Correct fertilizer dosing
✔ Reliable pharmaceuticals
✔ Efficient industrial processes
✔ Environmental protection
Errors distort concentration measurement and undermine system control.
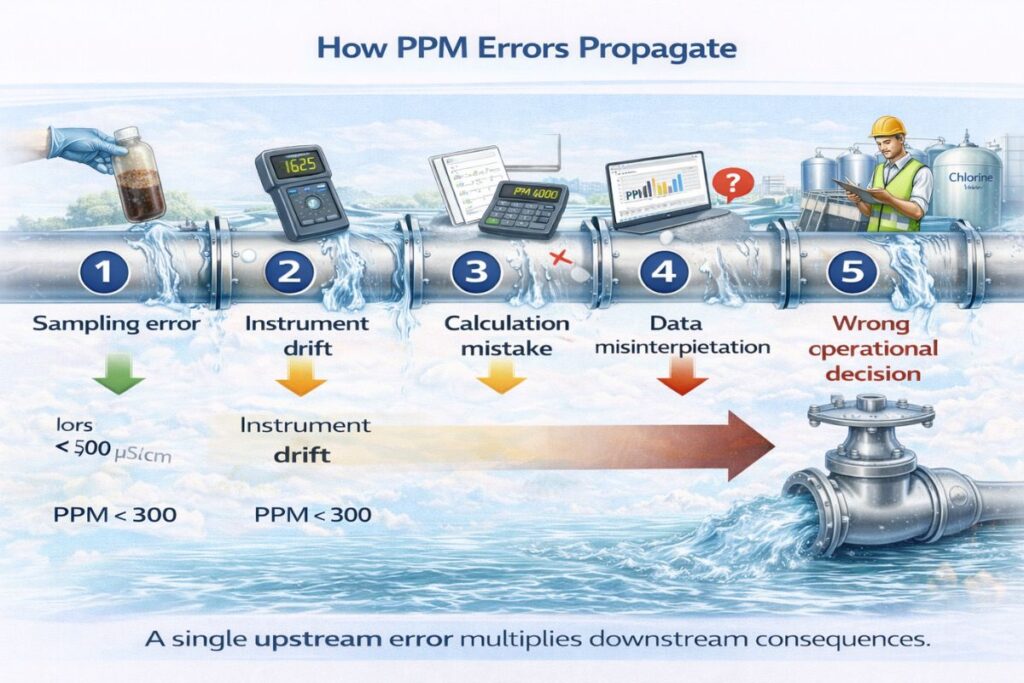
This is why eliminating ppm measurement mistakes is critical.
Top 10 PPM Measurement Mistakes — And How to Fix Them
1. Using Uncalibrated Instruments
The Problem
Meters and sensors drift over time, producing inaccurate readings.
Example
A TDS meter shows 350 ppm instead of actual 420 ppm.
Fix
✔ Calibrate weekly
✔ Use certified standards
✔ Maintain calibration logs
Case Study
A water utility reduced reporting errors by 42% after adopting monthly calibration schedules.
2. Confusing PPM with mg/L and mg/kg
The Problem
Users assume ppm is always equivalent to mg/L or mg/kg.
Reality
- Water: 1 ppm ≈ 1 mg/L
- Soil: 1 ppm ≈ 1 mg/kg
- Other liquids: Density matters
Fix
Use density corrections for non-water systems.
Calculation Example
Liquid density = 1.2 kg/L
Reading = 10 mg/L
PPM = 10 / 1.2 = 8.3 ppm
3. Ignoring Sample Contamination
The Problem
Dirty containers introduce foreign material.
Industry Example
Trace metals from steel containers inflated readings by 15%.
Fix
✔ Use acid-washed bottles
✔ Avoid metal tools
✔ Wear gloves
4. Incorrect Dilution Factor Application
The Problem
Forgetting to account for dilution during analysis.
Example
Original sample diluted 1:10
Measured = 5 ppm
Actual = 50 ppm
Fix
Always record dilution factors.
Formula
Actual PPM = Measured × Dilution Factor
5. Misapplying the PPM Formula
The Problem
Using incorrect mathematical relationships.
Standard PPM Formula
PPM = (Mass of Solute / Mass of Solution) × 1,000,000
Frequent Error
Dividing by volume instead of mass in solids.
Fix
Verify formula suitability for each matrix.
6. Overlooking Temperature Effects
The Problem
Temperature changes affect EC-based ppm readings.
Example
At 35°C, EC increases by ~7%.
Fix
✔ Use temperature-compensated meters
✔ Apply correction factors
7. Relying on Fixed EC-to-PPM Conversion Factors
The Problem
Using a universal factor (0.5 or 0.7) regardless of water type.
Reality
Different ions conduct differently.
Comparison Table
| Water Type | Conversion Factor |
|---|---|
| Drinking | 0.5 |
| Irrigation | 0.65 |
| Industrial | 0.7 |
Fix
Select factor based on water chemistry.
8. Inadequate Sampling Methods
The Problem
Non-representative samples distort results.
Visual Explanation
Sampling from surface only vs mixed-depth sampling.
Fix
✔ Composite sampling
✔ Multiple locations
✔ Consistent depth
9. Premature Rounding of Data
The Problem
Rounding too early reduces accuracy.
Example
Raw = 3.456 ppm
Rounded early → 3.5 ppm
Final error → ±1.3%
Fix
Round only in final reporting.
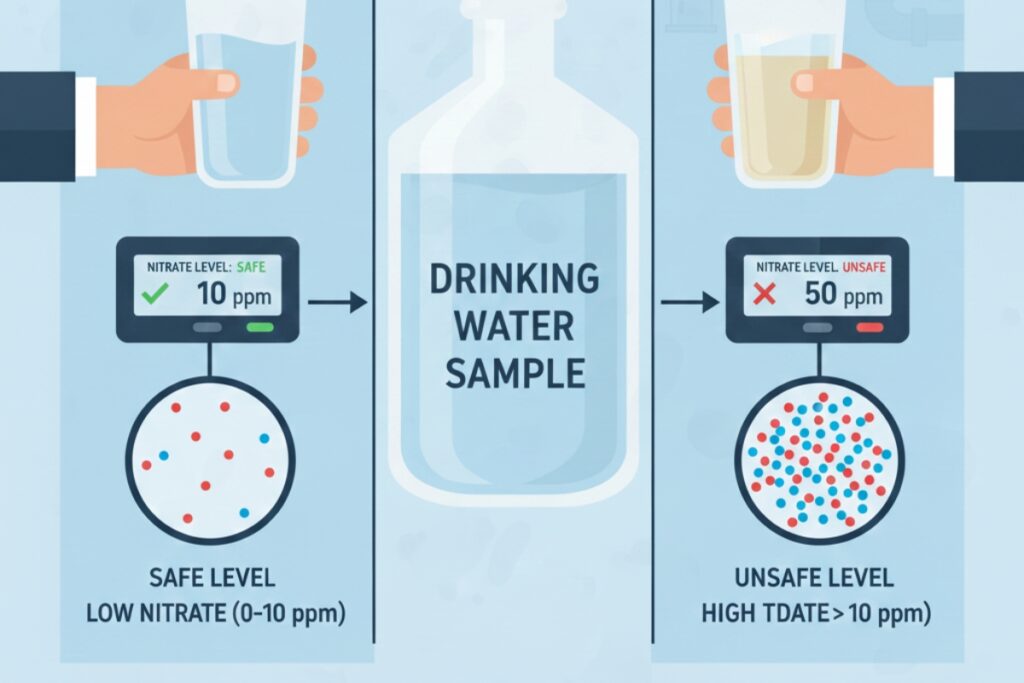
10. Misinterpreting Regulatory Limits
The Problem
Confusing ppb and ppm standards.
Example
Arsenic limit = 10 ppb (0.01 ppm)
Reported = 0.1 ppm → 10× unsafe
Fix
Always confirm unit basis.
Common PPM Errors vs Correct Practices
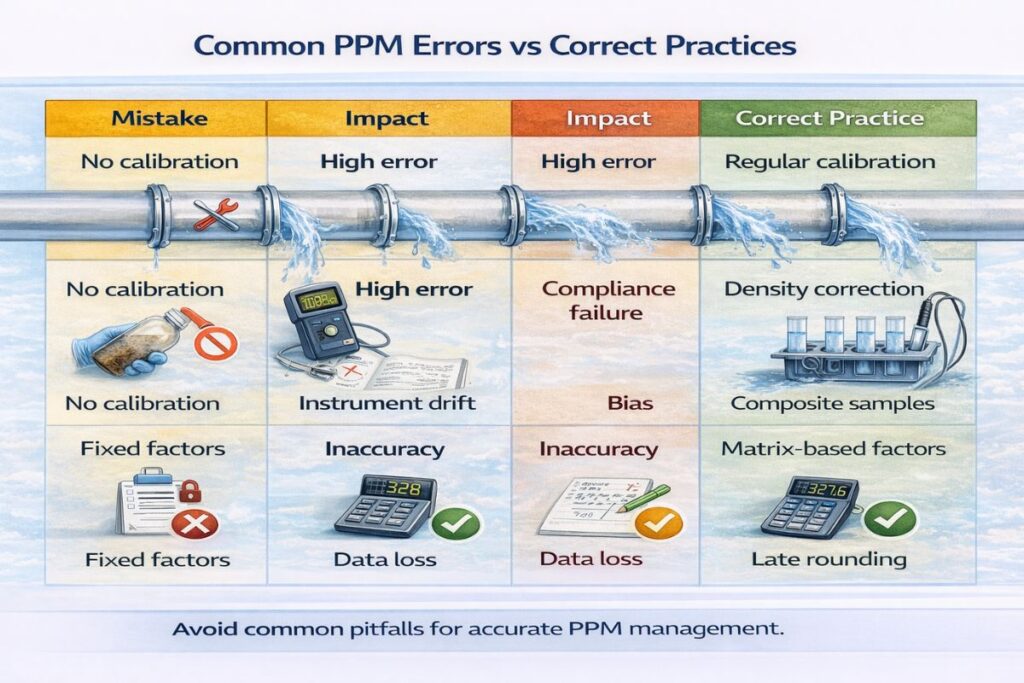
Comparison Table
| Mistake | Impact | Correct Practice |
|---|---|---|
| No calibration | High error | Regular calibration |
| Wrong units | Compliance failure | Density correction |
| Poor sampling | Bias | Composite samples |
| Fixed factors | Inaccuracy | Matrix-based factors |
| Early rounding | Data loss | Late rounding |
Calculation Walkthrough: Correcting a Faulty PPM Measurement
Scenario
Measured EC = 1,000 µS/cm
Used factor = 0.5
Temperature = 32°C
Actual factor = 0.65
Step 1: Temperature Correction (≈5%)
Corrected EC = 1,000 × 0.95 = 950
Step 2: Apply Proper Factor
PPM = 950 × 0.65 = 618 ppm
Original Report
500 ppm (incorrect)
Correct Value
618 ppm (accurate)
Error = 23.6%
Industry Case Study: Pharmaceutical Batch Rejection
Background
A pharma company reported solvent residue = 8 ppm.
Limit = 10 ppm
Audit found:
- Dilution factor ignored
- Actual = 16 ppm
Impact
- Batch rejected
- ₹2.5 crore loss
Resolution
Standardized ppm calculation procedures and digital tools.
Tools & Calculators for Error-Free PPM Measurement
Manual calculation increases risk. Digital tools reduce human error.
Trusted Platform: ppmcalculation.com
ppmcalculation.com provides professional-grade tools for accurate measurement:
Advantages
- Validated ppm formulas
- Automatic corrections
- Mobile-friendly interface
- No registration
- Audit-ready outputs
These tools significantly reduce ppm measurement mistakes.
Common Mistakes Recap Checklist
Before finalizing any ppm report, verify:
✔ Instrument calibrated
✔ Units validated
✔ Sample uncontaminated
✔ Dilution applied
✔ Temperature corrected
✔ Proper factor used
✔ Rounding delayed
Use this checklist for quality control.
Frequently Asked Questions (FAQs)
1. What is the most common ppm measurement mistake?
Skipping calibration is the most frequent and damaging error.
2. Can ppm errors affect regulatory compliance?
Yes. Even minor deviations can lead to violations.
3. Are cheap meters reliable?
Only for screening. Professional work requires certified instruments.
4. Why do lab and field results differ?
Due to temperature, contamination, and calibration differences.
5. How often should ppm instruments be calibrated?
Weekly for critical systems, monthly for routine monitoring.
6. Is ppm always better than ppb?
No. Toxic contaminants often require ppb-level analysis.
Eliminating PPM Measurement Mistakes for Reliable Results
Avoiding ppm measurement mistakes is essential for scientific integrity, safety, and compliance.
Key Takeaways
✔ Calibration is non-negotiable
✔ Use correct ppm formula
✔ Account for density and temperature
✔ Apply dilution factors
✔ Interpret limits carefully
✔ Use validated digital tools
Systematic error control ensures trustworthy concentration measurement.
Related PPM Calculators
Explore more water quality and chemistry tools: