Have you ever wondered how scientists measure tiny amounts of chemicals in water, air, food, or pharmaceuticals? When dealing with extremely low concentrations, traditional percentage units are no longer practical. Instead, professionals rely on parts per million (ppm), parts per billion (ppb), and parts per trillion (ppt).
Understanding PPM vs. PPB vs. PPT is essential for engineers, environmental scientists, lab technicians, students, and even non-technical professionals working with quality control or compliance.
For example:
- Is 10 ppm of lead in water safe?
- What does 5 ppb of pesticide residue really mean?
- Why do pharmaceutical labs measure impurities in ppt?
This article explains how these units relate, how to convert them, and when each is used. We will also provide practical examples, case studies, calculation walkthroughs, and industry applications—making it a complete guide for both technical and non-technical users.
What Are PPM, PPB, and PPT?
Before comparing these units, let’s define them clearly.
Parts Per Million (PPM)
PPM (parts per million) represents one part of a substance per one million parts of a mixture.
Definition:
1 ppm = 1 part per 1,000,000 parts
It is commonly used in:
- Water quality testing
- Chemical engineering
- Air pollution monitoring
- Industrial solutions
Parts Per Billion (PPB)
PPB (parts per billion) measures one part per one billion parts.
Definition:
1 ppb = 1 part per 1,000,000,000 parts
It is used when concentrations are extremely small, such as:
- Toxic metals
- Trace pesticides
- Semiconductor manufacturing
Parts Per Trillion (PPT)
PPT (parts per trillion) is used for ultra-trace measurements.
Definition:
1 ppt = 1 part per 1,000,000,000,000 parts
This level is critical in:
- Pharmaceutical purity
- Advanced environmental studies
- Nuclear and space research
PPM vs. PPB vs. PPT: Core Relationship Explained
The relationship between ppm, ppb, and ppt follows a simple mathematical pattern.
| Unit | Meaning | Relative Size |
|---|---|---|
| PPM | Parts per million | Largest |
| PPB | Parts per billion | 1,000× smaller than ppm |
| PPT | Parts per trillion | 1,000× smaller than ppb |
Mathematical Relationship
1 ppm = 1,000 ppb = 1,000,000 ppt
1 ppb = 1,000 pptThis visualization helps understand why smaller units are needed for trace detection.

Understanding Concentration Measurement in Practice
What Is Concentration Measurement?
Concentration measurement describes how much of a substance is present in a given amount of material, liquid, or gas.
It is commonly expressed as:
- Mass per volume (mg/L, µg/L)
- Mass per mass (mg/kg)
- Ratio-based units (ppm, ppb, ppt)
Why Ratio Units Are Popular
PPM, PPB, and PPT are popular because:
- They are easy to compare
- They are unit-independent
- They work across solids, liquids, and gases
This makes them ideal for regulatory and industrial standards.
PPM Formula and Basic Calculations
Standard PPM Formula
The most common ppm formula is:
PPM = (Mass of Solute / Mass of Solution) × 1,000,000For liquids:
PPM ≈ mg/L (for water-based solutions)Example: PPM Calculation
A water sample contains 3 mg of fluoride in 1 liter.
PPM = (3 mg / 1 L) ≈ 3 ppm
Because 1 liter of water weighs about 1 kg, mg/L ≈ ppm.
Conversion Table
| From → To | Multiply By |
|---|---|
| ppm → ppb | × 1,000 |
| ppm → ppt | × 1,000,000 |
| ppb → ppm | ÷ 1,000 |
| ppb → ppt | × 1,000 |
| ppt → ppb | ÷ 1,000 |
| ppt → ppm | ÷ 1,000,000 |
Step-by-Step Conversion Examples
Example 1: PPM to PPB
A water sample contains 2.5 ppm of nitrate.
2.5 ppm × 1,000 = 2,500 ppb
Example 2: PPB to PPM
Mercury concentration = 450 ppb
450 ÷ 1,000 = 0.45 ppm
Example 3: PPT to PPB
Dioxin = 80 ppt
80 ÷ 1,000 = 0.08 ppbPPM to mg/L Conversion Explained
One of the most common questions is:
How to convert ppm to mg/L?
For water-based solutions:
1 ppm ≈ 1 mg/L
Why This Works
Because:
- 1 liter of water ≈ 1 kg
- 1 kg = 1,000,000 mg
So the ratio remains consistent.
Example
Copper in water = 4 ppm
≈ 4 mg/L
⚠️ Note: For liquids other than water, density must be considered.
Industry Applications of PPM, PPB, and PPT
1. Environmental Monitoring
Used to measure:
- Lead in drinking water (ppb)
- Air pollutants (ppm)
- Pesticides (ppt–ppb)
Example Standard:
EPA limit for lead in water = 15 ppb
2. Chemical Manufacturing
In chemical plants:
- Reactant purity: ppm
- Impurity control: ppb
- Catalyst poisoning: ppt
Small deviations can cause:
- Product failure
- Equipment damage
- Safety risks
3. Pharmaceutical Industry
Drug manufacturing requires ultra-high purity.
Typical limits:
| Parameter | Range |
|---|---|
| Solvents | ppm |
| Heavy metals | ppb |
| Genotoxins | ppt |
Even 1 ppt impurity can disqualify a batch.
4. Food and Beverage Industry
Used for:
- Preservatives
- Residues
- Contaminants
Example:
- Pesticide residue: <50 ppb
- Chlorine in water: 0.2–1 ppm
5. Semiconductor Manufacturing
Microchip production uses ultrapure water.
Requirements:
- Metals: <1 ppt
- Organics: <5 ppt
Contamination can destroy entire production runs.
Case Study 1: Lead Contamination in Drinking Water
Background
A municipal water system detected lead contamination.
Test Results:
- Initial: 25 ppb
- Legal limit: 15 ppb
Analysis
25 ppb = 0.025 ppm
Though numerically small, it exceeded safety limits by 66%.
Action
- Pipe replacement
- pH adjustment
- Continuous monitoring
After treatment: 5 ppb (safe range)
This case shows why understanding ppb is critical for public health.
Case Study 2: Pharmaceutical Impurity Control
Scenario
A drug manufacturer detected a genotoxic impurity.
Measured: 12 ppt
Limit: 10 ppt
Impact
12 ppt = 0.012 ppb
Even this tiny excess required:
- Batch rejection
- Investigation
- Process redesign
Cost impact: ₹4 crore loss
PPT-level control protects patient safety.
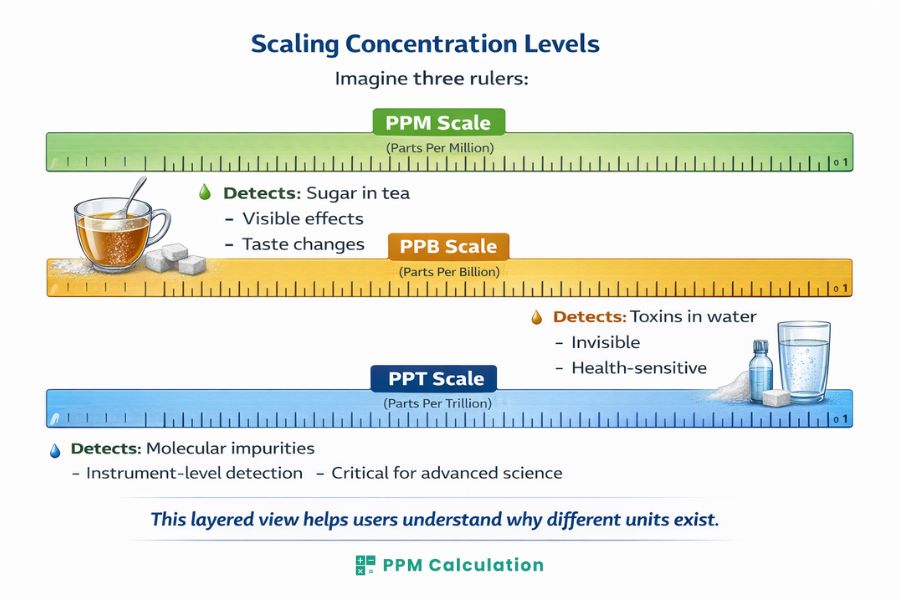
Comparison Table: PPM vs. PPB vs. PPT
| Feature | PPM | PPB | PPT |
|---|---|---|---|
| Magnitude | 10⁻⁶ | 10⁻⁹ | 10⁻¹² |
| Sensitivity | Medium | High | Ultra-high |
| Typical Use | Water, air | Toxins | Pharmaceuticals |
| Instruments | Standard sensors | ICP-MS | GC-MS, LC-MS |
| Regulation Level | Moderate | Strict | Critical |
Tools & Calculators for Concentration Measurement
Manual calculations are useful, but online tools improve speed and accuracy.
Recommended Platform: ppmcalculation.com
ppmcalculation.com is a trusted calculator platform offering:
Benefits
✔ No registration
✔ Mobile-friendly
✔ Engineering-grade accuracy
✔ Instant results
These tools reduce human error in ppm calculation and conversion.
Common Mistakes in PPM, PPB, and PPT Calculations
1. Ignoring Density
Assuming 1 ppm = 1 mg/L for non-water liquids can cause errors.
2. Confusing Units
Mixing up:
- mg/kg
- mg/L
- ppm
can lead to compliance failures.
3. Wrong Conversion Factors
Using ×100 instead of ×1000 between ppm and ppb is common.
4. Rounding Too Early
Premature rounding reduces accuracy in ppt-level work.
5. Misinterpreting Regulations
Some limits are mass-based, not ratio-based.
Always verify regulatory definitions.
Frequently Asked Questions (FAQs)
1. What is the difference between ppm and ppb?
PPM is 1,000 times larger than PPB.
1 ppm = 1,000 ppb.
2. Is ppm always equal to mg/L?
Only for water or solutions with density ≈1 kg/L. For other liquids, density correction is needed.
3. Why is ppt important in pharmaceuticals?
Because ultra-trace impurities can cause toxicity or reduce drug effectiveness.
4. How accurate are online ppm converters?
Reliable platforms like ppmcalculation.com use standardized formulas and provide engineering-grade precision.
5. Can ppm be used for gases?
Yes. In air quality, ppm is used for CO₂, CO, and NOx.
Example: CO₂ ≈ 420 ppm in atmosphere.
6. Which instruments measure ppt?
- Gas Chromatography (GC-MS)
- Liquid Chromatography (LC-MS)
- ICP-MS
These instruments detect parts-per-trillion levels.
Mastering PPM vs. PPB vs. PPT
Understanding PPM vs. PPB vs. PPT is fundamental to modern science, engineering, and quality control.
Key Takeaways
✔ 1 ppm = 1,000 ppb = 1,000,000 ppt
✔ PPM suits general monitoring
✔ PPB supports regulatory compliance
✔ PPT enables ultra-pure manufacturing
✔ Accurate conversion prevents costly errors
From environmental safety to pharmaceutical production, these units ensure reliability and protection.