Advanced PPM → PPB Converter
Convert PPM to PPB precisely. This tool accepts plain numbers or scientific notation (e.g. 1.2e-3). Choose display precision and copy the result with one click.
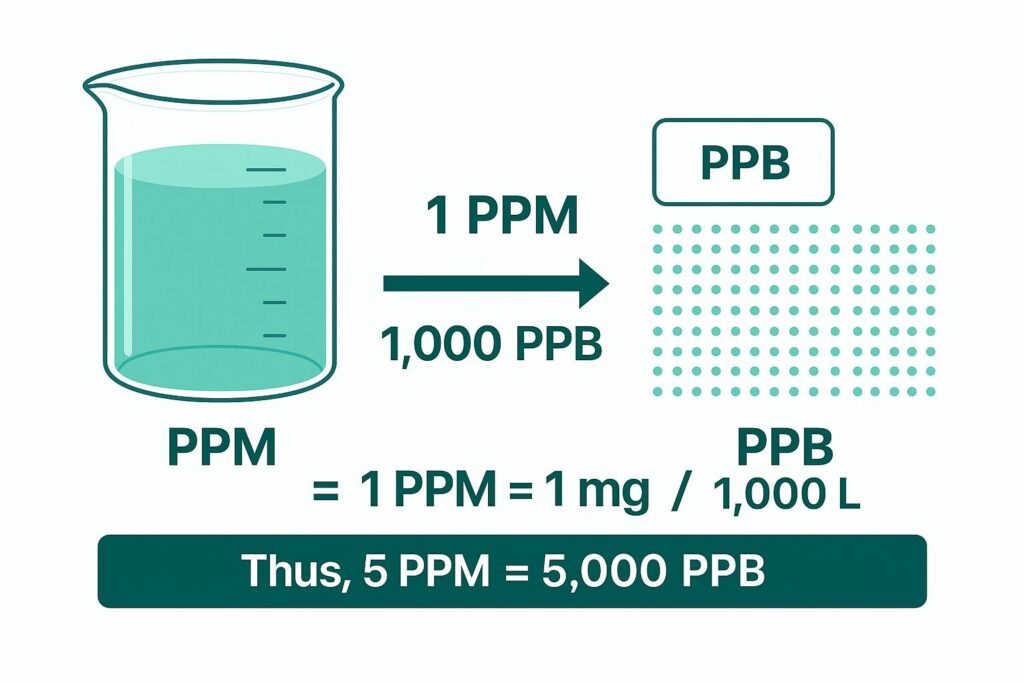
PPB = PPM × 1000
If a water sample contains 5 PPM of a contaminant:
5 × 1000 = 5,000 PPB
This means 5 parts of a substance per million becomes 5,000 parts per billion.
PPM to PPB Conversion
Concentration measurements in environmental science, analytical chemistry, toxicology, hydrology, and industrial quality control frequently operate at extremely small scales. Two of the most universal measurement units in these fields are parts per million (PPM) and parts per billion (PPB). Although they appear similar, these units reflect vastly different concentration levels, and misunderstanding their relationship can lead to serious reporting errors or regulatory non-compliance.
What PPM and PPB Actually Represent at the Molecular Level
Both PPM and PPB quantify how many “parts” of a substance exist per total “parts” of solution or mixture.
- 1 PPM = 1 part solute per 1,000,000 parts solution
- 1 PPB = 1 part solute per 1,000,000,000 parts solution
For dilute aqueous solutions:
- 1 PPM ≈ 1 mg per liter
- 1 PPB ≈ 1 µg per liter
This is why PPB is considered a micro-trace measurement.
It is often used for contaminant detection, pollutant monitoring, and toxicological studies where extremely low concentrations must be accurately expressed.
Mathematical Relationship Between PPM and PPB
Because “billion” is exactly 1000 times larger than “million,” the conversion is linear:
PPB = PPM × 1000
This ratio remains valid regardless of whether the concentration is expressed by:
- mass/mass
- mass/volume
- volume/volume
- molar ratios (in highly dilute systems)
This is why the conversion is universal across environmental, industrial, and laboratory domains.
Why Scientists Convert PPM to PPB
Analysts rarely report PPM when the concentration becomes too small to meaningfully represent. PPB gives better resolution and more intuitive interpretation.
PPB is preferred when:
- contaminants are near regulatory limits
- metals or toxins are detected in groundwater
- VOCs are measured in indoor environments
- pesticide residues or chemical traces are analyzed
- highly sensitive instruments like ICP-MS or GC-MS are used
Using PPB instead of PPM avoids rounding errors and provides clarity when working at micro-concentration levels.
Real Scientific Examples of PPM → PPB Conversion
Example 1 — Lead contamination in drinking water
Measured concentration: 0.015 PPM
PPB = 0.015 × 1000 = 15 PPB
EPA regulations often specify lead limits in PPB, not PPM.
Example 2 — Pesticide traces in vegetables
Lab result: 0.9 PPM of residue
PPB = 0.9 × 1000 = 900 PPB
Food safety agencies typically report thresholds in PPB because residues are extremely small.
Example 3 — Indoor air quality (VOCs)
Formaldehyde detected: 0.12 PPM
PPB = 0.12 × 1000 = 120 PPB
Most indoor air guidelines specify VOC limits in PPB because VOC effects occur at extremely low concentrations.
Interpreting PPM and PPB in Practical Scenarios
Water quality:
- Arsenic limits are often 10 PPB, not PPM
- Mercury safety thresholds are near 2 PPB
Food chemistry:
- Residual solvents are regulated in the 100–1000 PPB range
Environmental science:
- River contaminants are frequently below 1 PPM, requiring PPB conversion
Toxicology:
- Biological exposure modeling often uses PPB for dose-response curves
Switching from PPM to PPB can significantly improve how clearly these risks and concentrations are communicated.
