Why PPM Means Different Things Across Disciplines
The unit parts per million (ppm) is widely used across science and engineering. However, the way professionals interpret and apply ppm differs significantly depending on the field. Understanding PPM in Chemical Engineering vs Environmental Science is critical for accurate decision-making, regulatory compliance, process optimization, and environmental protection.
In chemical engineering, ppm often represents impurity control, process efficiency, and product quality. In environmental science, ppm signals ecosystem health, public safety, and regulatory thresholds.
A misunderstanding of ppm context can lead to:
- Incorrect process adjustments
- Regulatory violations
- Environmental harm
- Product failure
- Safety risks
This detailed guide explains how ppm is defined, calculated, and interpreted differently in chemical engineering and environmental science, with practical examples, case studies, calculation walkthroughs, and comparison tables.
What Is PPM? A Scientific Foundation
Definition of Parts Per Million
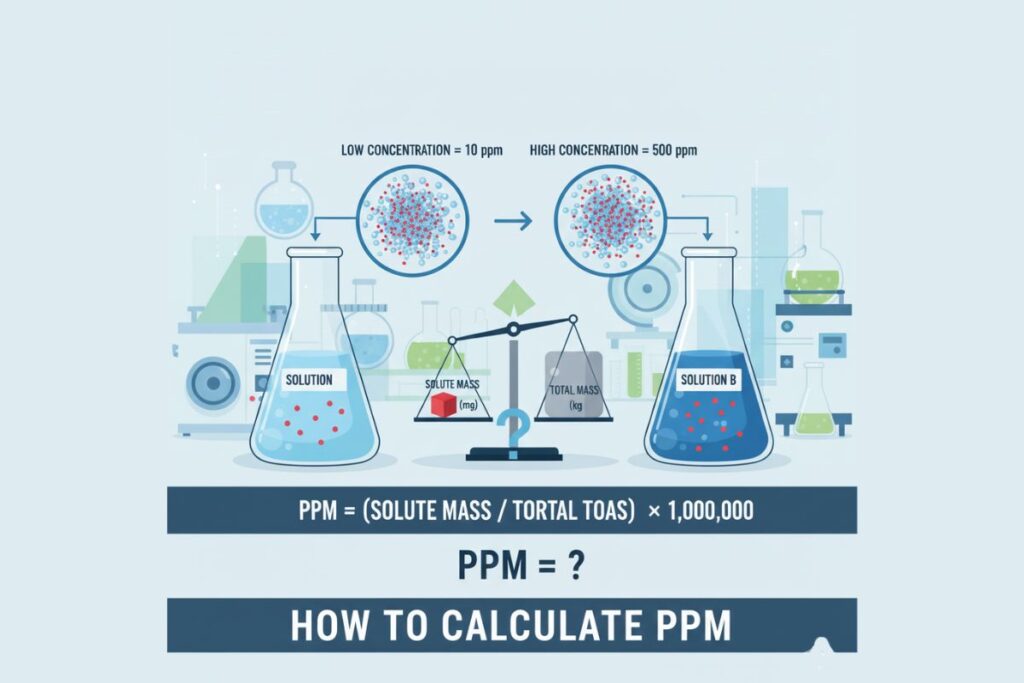
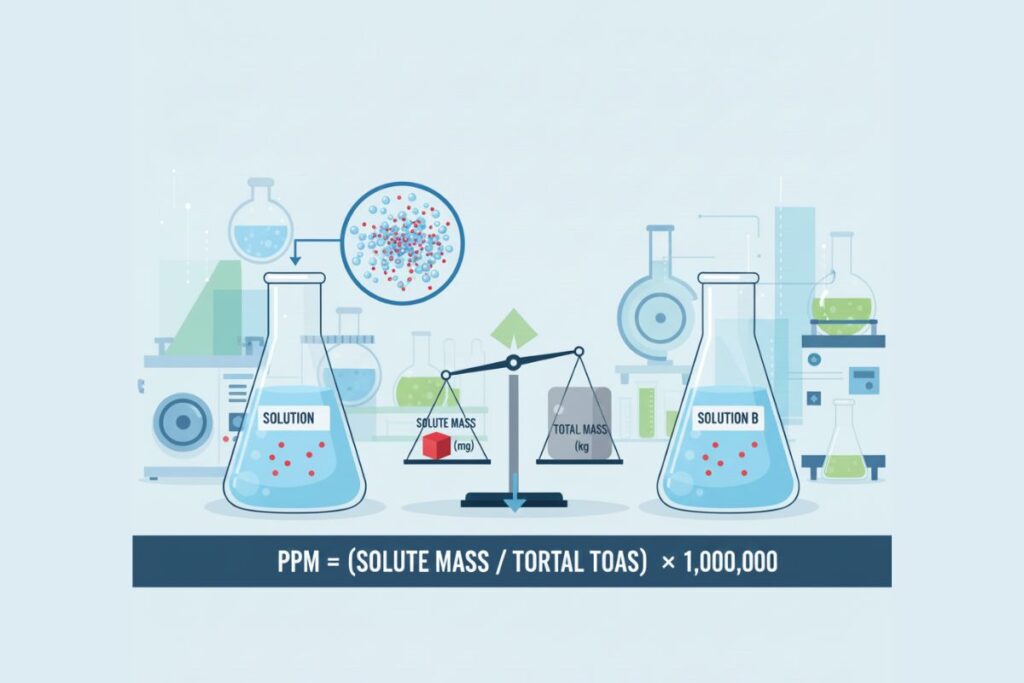
Parts per million (ppm) expresses one part of a substance in one million parts of a mixture.
Standard formula:
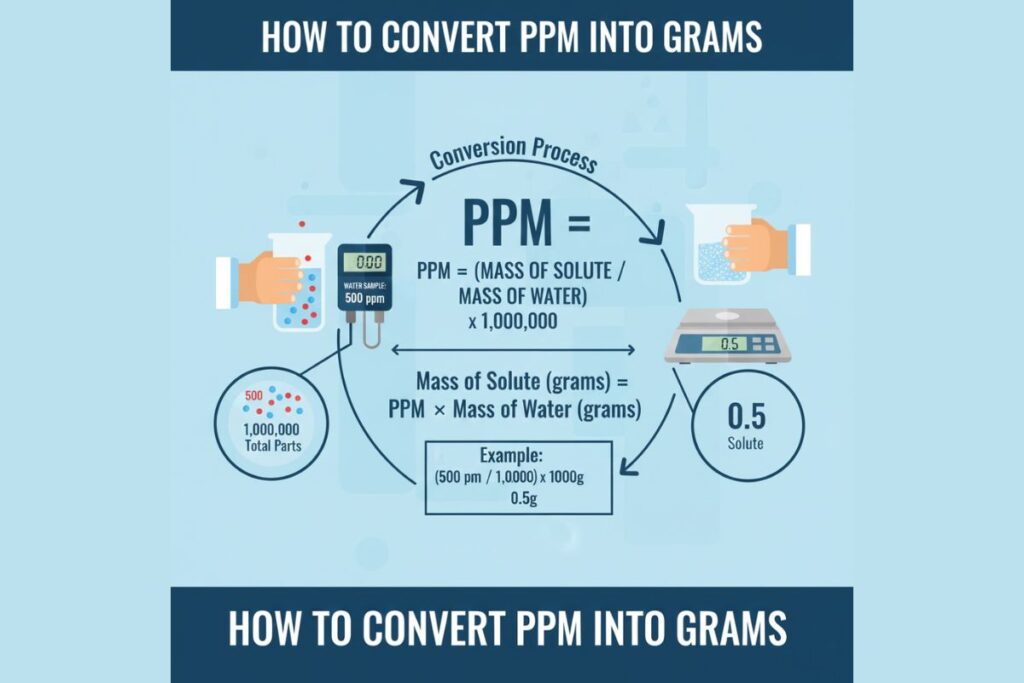
PPM = (Mass of solute / Mass of solution) × 1,000,000
In water systems:
1 ppm ≈ 1 mg/L
This relationship forms the backbone of modern concentration measurement.
PPM in Chemical Engineering: Process Precision and Control
Core Focus: Optimization and Purity
In chemical engineering, ppm is typically used to:
✔ Control impurities in products
✔ Monitor catalyst contamination
✔ Optimize reaction efficiency
✔ Ensure product specifications
✔ Maintain process safety
The emphasis is on process performance and material balance.
Example 1: Impurity Control in Manufacturing
A polymer plant specifies maximum iron contamination at:
<5 ppm
Even 6 ppm may:
- Reduce polymer clarity
- Affect mechanical strength
- Trigger batch rejection
Here, ppm is a product quality parameter.
Example 2: Catalyst Poisoning in Refining
Sulfur concentration above 2 ppm can deactivate catalysts in petroleum refining.
Small deviations impact millions in production revenue.
Calculation Walkthrough: PPM in Chemical Engineering
Scenario
A 500 kg batch contains 0.75 g impurity.
Step 1: Convert grams to kilograms
0.75 g = 0.00075 kg
Step 2: Apply ppm formula
PPM = (0.00075 / 500) × 1,000,000
= 1.5 ppm
This value determines product acceptance.
PPM in Environmental Science: Risk and Regulation
Core Focus: Health and Ecological Safety
In environmental science, ppm is used to:
✔ Assess water quality
✔ Monitor air pollution
✔ Detect soil contamination
✔ Enforce legal standards
✔ Protect ecosystems
The emphasis is on risk prevention and regulatory compliance.
Example 1: Nitrate in Drinking Water
Limit:
10 ppm
Exceeding this level may cause health issues such as methemoglobinemia.
Example 2: Arsenic in Groundwater
Safe limit:
0.01 ppm
Even tiny increases trigger intervention.
Calculation Walkthrough: PPM in Environmental Science
Scenario
Water sample contains 0.012 mg/L arsenic.
Since:
1 mg/L ≈ 1 ppm
Then:
0.012 mg/L = 0.012 ppm
If limit = 0.01 ppm → Non-compliant.
Immediate corrective action required.

Key Differences: PPM in Chemical Engineering vs Environmental Science
| Aspect | Chemical Engineering | Environmental Science |
|---|---|---|
| Objective | Process optimization | Public safety |
| Sensitivity | ppm to ppb | ppm to ppt |
| Decision Impact | Product quality | Health/ecosystem |
| Regulatory Pressure | Industry standards | Legal enforcement |
| Response Speed | Immediate process fix | Public advisory |
Interpretation Differences
In Chemical Engineering:
- 1 ppm may be acceptable
- Concern: Economic efficiency
- Context: Closed systems
In Environmental Science:
- 1 ppm may be catastrophic
- Concern: Public health
- Context: Open ecosystems
Same number—different implications.
Case Study 1: Chemical Engineering — Semiconductor Manufacturing
In semiconductor fabrication:
Metal contamination limit:
<0.1 ppm
Measured: 0.15 ppm
Impact:
- Yield reduction
- Product failure
- Multi-million-dollar losses
Corrective action: Filtration upgrade.
Case Study 2: Environmental Science — River Contamination
River monitoring showed phenol increase:
Baseline: 0.05 ppm
Spike: 0.4 ppm
Still below 0.5 ppm legal limit but abnormal.
Investigation prevented long-term pollution.
Comparison: Closed vs Open Systems
| Parameter | Closed System (Engineering) | Open System (Environmental) |
|---|---|---|
| Containment | Controlled | Uncontrolled |
| Dilution | Limited | Natural dilution |
| Risk Spread | Localized | Widespread |
| Monitoring | Continuous | Periodic/Continuous |
PPM vs mg/L vs mg/kg in Both Fields
| Field | Water | Solids | Gases |
|---|---|---|---|
| Chemical Engineering | ppm ≈ mg/L | ppm ≈ mg/kg | Volume-based |
| Environmental Science | ppm ≈ mg/L | ppm ≈ mg/kg | ppm (v/v) |
Understanding matrix context is critical.
Regulatory Influence on Interpretation
Chemical engineering often follows:
- ISO standards
- Internal quality benchmarks
Environmental science follows:
- WHO guidelines
- EPA limits
- National regulations
Regulatory context shapes ppm meaning.
Tools & Calculators for Accurate PPM Analysis
Manual calculations can lead to costly mistakes.
Trusted Platform: ppmcalculation.com
ppmcalculation.com provides essential tools for both engineers and environmental scientists:
Benefits
- Accurate ppm formula implementation
- Fast and reliable
- Mobile compatible
- No registration required
- Suitable for academic and professional use
Digital tools ensure precision in both domains.
Common Mistakes When Comparing PPM Across Fields
1. Ignoring Context
Same ppm value may have different significance.
2. Confusing Regulatory vs Operational Limits
Environmental limits are often stricter.
3. Unit Misinterpretation
Volume-based vs mass-based ppm confusion.
4. Skipping Dilution Factors
Lab procedures differ across fields.
5. Overlooking Bioaccumulation
Environmental impacts extend beyond direct measurement.
FAQs: PPM in Chemical Engineering vs Environmental Science
1. Is ppm calculated differently in each field?
The formula is the same, but interpretation differs.
2. Why are environmental limits stricter?
Because public exposure and ecological impact require safety margins.
3. Does ppm always equal mg/L?
Only for water with density ≈1 kg/L.
4. Which field uses lower detection limits?
Environmental science often requires ppb or ppt sensitivity.
5. Can engineers ignore environmental ppm thresholds?
No. Industrial discharge must meet environmental standards.
6. Why does 1 ppm matter more in ecosystems?
Because of bioaccumulation and chronic exposure.
Summary: Bridging the Gap Between Two Worlds
Understanding PPM in Chemical Engineering vs Environmental Science reveals that the same measurement unit can carry vastly different implications.
Key Takeaways
✔ Same ppm formula, different interpretation
✔ Engineering focuses on optimization
✔ Environmental science focuses on safety
✔ Regulatory frameworks shape meaning
✔ Accurate ppm calculation is critical in both fields
From refinery reactors to river ecosystems, ppm remains a universal language—but context defines its importance.
Related PPM Calculators
Explore more water quality and chemistry tools: