PPM Formula Explained
The PPM formula is a scientific method used to calculate parts per million when measuring extremely small concentrations of substances in air, water, soil, food, chemicals, and industrial processes. PPM (parts per million) expresses how many units of a substance exist per 1,000,000 units of the total mixture. The formula used depends on whether the measurement applies to liquid solutions, gas concentrations, or mass-based samples. Understanding the correct PPM calculation formula allows accurate environmental monitoring, laboratory testing, and safety compliance.
What Does PPM Represent
PPM concentration represents a mixing ratio and is used whenever amounts are too small to measure in percentages. Instead of writing 0.0001%, scientists write 1 PPM, which is easier to compare. 1 PPM represents 1 part of substance per 1 million parts of mixture. It is used for pollutants, dissolved solids, trace chemicals, nutrients, and gases that may have major effects even at low levels.
General PPM Formula
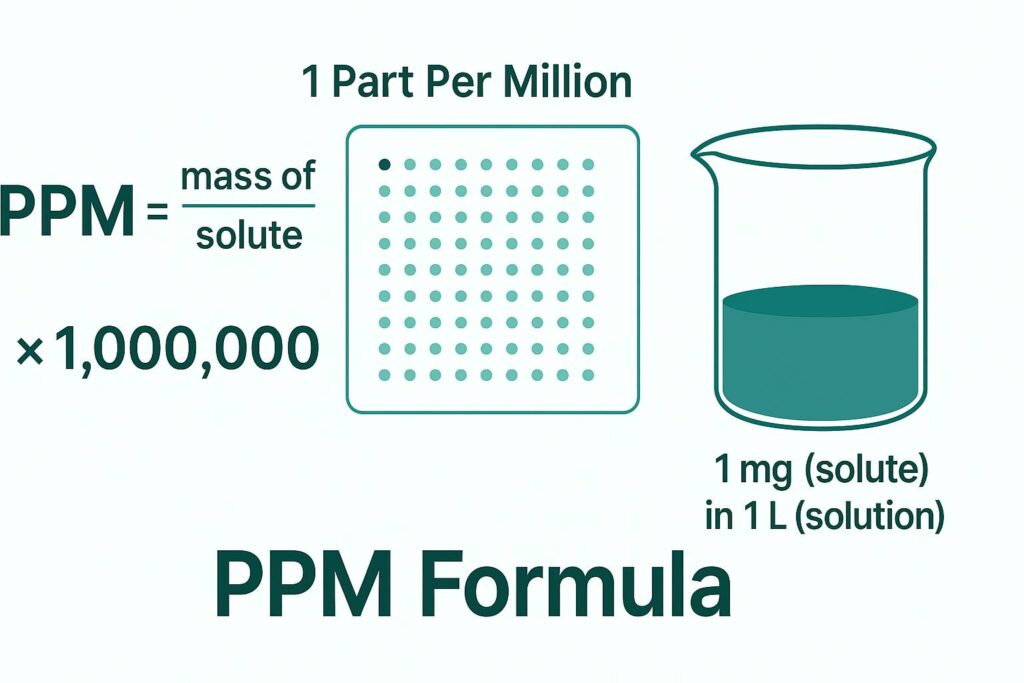
The most general scientific expression of the PPM formula is:
PPM = (Mass of Solute ÷ Mass of Solution) × 1,000,000
This represents the basic definition of PPM across all scientific fields. It is used when both solute and solution masses are known. The multiplication by 1,000,000 converts the ratio into parts per million.
Example:
If 0.002 g of contaminant is dissolved in 2 kg of soil:
PPM = (0.002 ÷ 2000) × 1,000,000 = 1 PPM
This method is useful in agriculture, soil chemistry, and industrial contamination testing.
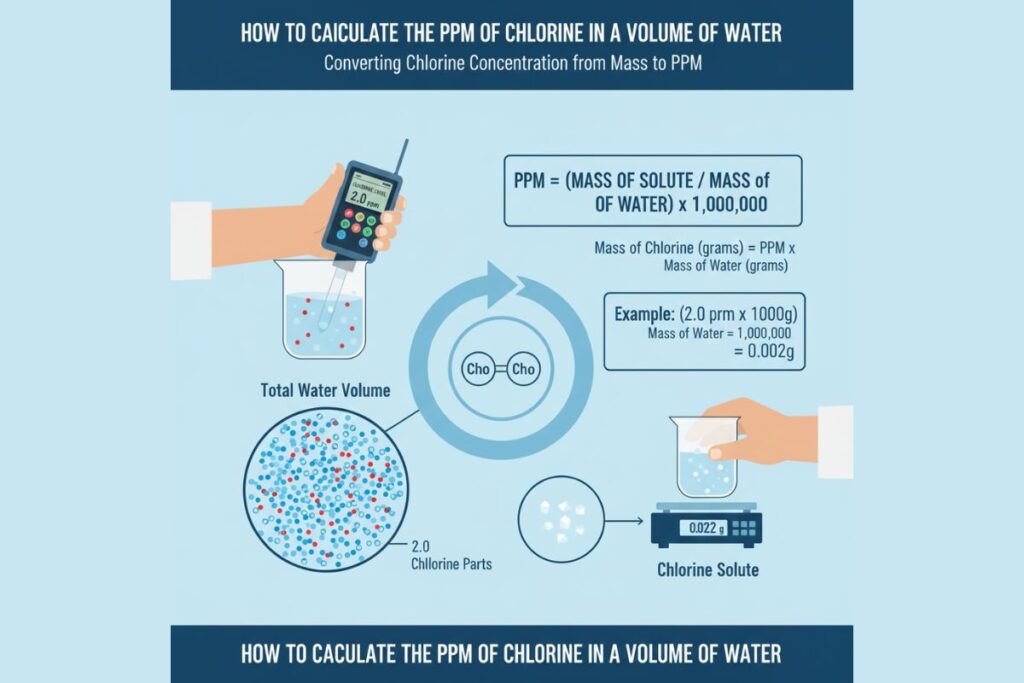
PPM Formula in Water: mg/L
In water-based chemistry, the most common PPM calculation is related to mg/L.
PPM = mg of solute per litre of water
This works because 1 L of water = 1,000,000 mg, allowing a 1:1 conversion. This version is widely used in water quality, aquaculture, food processing, wastewater treatment, and pool chemistry.
Example:
If a water sample contains 3 mg of chlorine per litre:
PPM = 3 mg/L = 3 PPM
Keywords used: PPM in water, PPM in liquids, drinking water standards, water chemistry PPM.
PPM Formula in Gases: Ideal Gas Law
Gas concentration uses a more advanced PPM formula because gases change volume depending on temperature and pressure. Scientists use the ideal gas law to calculate gas PPM accurately.
PPM = (Mass Concentration × R × T) ÷ (MW × P × 1000)
Where:
R = 0.082057 L·atm·mol⁻¹·K⁻¹
T = temperature in Kelvin
MW = molecular weight
P = pressure in atm
This formula ensures accurate air quality measurement based on actual environmental conditions.
Example for ozone concentration:
50 µg/m³ of O₃ at 25°C and 1 atm
PPM = approx 0.025 PPM
Keywords used: PPM in air, gas concentration calculation, air pollution monitoring, ideal gas law.
PPM Formula for Volume-Based Gas Ratio
Sometimes PPM for gases is simply based on molecular fraction rather than mass:
PPM = Mole fraction × 1,000,000
This expresses how many molecules of a gas exist for every one million molecules of air. This method is used for CO₂ sensors, indoor air quality, and gas detector calibration.
Example:
If the mole fraction of CO₂ in indoor air is 0.0008:
PPM = 0.0008 × 1,000,000 = 800 PPM
This application is popular in ventilation design, HVAC, and workplace safety.
PPM Formula for Solutions by Volume
For liquid mixing based on volume:
PPM = (Volume of solute ÷ Volume of solution) × 1,000,000
Example:
A chemical additive of 0.7 mL is mixed into 70 L of solvent:
PPM = (0.7 ÷ 70000) × 1,000,000 = 10 PPM
Useful in industrial solvents, fuel additives, food chemistry.
Conversions Related to PPM
Because PPM is a mid-scale concentration unit, converting between PPM, percentage, and PPB (parts per billion) is common.
PPM = % × 10,000
PPB = PPM × 1,000
1 PPM = 0.0001%
1 PPM = 1000 PPB
These relationships strengthen scientific data reporting and ensure consistent measurements across sectors.
Why PPM Formula Is Crucial in Environmental Science
Many pollutants are toxic even at very low concentrations. Using PPM standards helps set air quality regulations, drinking water limits, and public health guidelines. PPM calculation is therefore vital in controlling:
• Nitrogen dioxide (NO₂)
• Ozone (O₃)
• Carbon monoxide (CO)
• Sulphur dioxide (SO₂)
• Volatile organic compounds (VOCs)
• Heavy metals in river water
• Chlorine and minerals in drinking water
These values influence environmental compliance, lab analysis, and industrial safety.
Scientific Examples Showing the Importance of PPM Formula
A CO₂ sensor detects 1200 PPM, signaling poor ventilation. If it rises above 1500 PPM, cognitive performance decreases.
A wastewater facility measures 10 PPM ammonia. Treatment continues until ammonia drops below 1 PPM.
A food laboratory detects 2 PPM sodium benzoate in fruit juice, verifying preservative limits.
Each of these decisions depends on accurate PPM formula calculations.
PPM in Soil Analysis
Soil chemistry uses PPM to determine nutrient content, contamination, and micronutrient deficiencies. If 8 mg of iron is found per kg of soil, the concentration is 8 PPM, helping farmers manage fertilizer amounts scientifically.
Soil PPM formula:
PPM = mg/kg = PPM concentration of nutrient in soil
Keywords: soil testing PPM, agriculture nutrient measurement, soil contamination.
Challenges in Using PPM Formula
Although PPM calculation seems simple, several conditions impact accuracy:
Temperature variations change gas PPM
Water density changes with salinity and dissolved solids
Measurement errors occur due to instrument limitations
Complex mixtures require mole-based PPM formulas
Understanding context ensures correct interpretation of PPM values.
PPM vs Other Measurement Units
PPM is more precise than percentage but less precise than PPB (parts per billion). It is commonly used when concentrations are neither extremely high nor extremely low. Because it fits the most common ranges found in water quality and air monitoring, PPM is the preferred scientific concentration unit.
Summary
The PPM formula provides a universal way to measure extremely small concentrations in liquids, gases and solid mixtures. It converts real-world samples into scientifically meaningful data that can be compared with health standards, environmental regulations and industrial tolerances. Whether calculating PPM in water using mg/L, PPM in air using the ideal gas law, or PPM in soil using mass ratios, understanding the correct formula is essential for accurate results. The parts per million calculation supports decision-making in public health, environmental protection, engineering and laboratory science, making it one of the most important measurement tools used today.