In chemistry, environmental science, industrial processing, and air quality monitoring, the unit parts per million (ppm) is widely used. However, many professionals and students misunderstand a crucial distinction: PPM by Volume vs PPM by Mass.
Using the wrong interpretation can lead to:
- Incorrect ppm calculation
- Regulatory non-compliance
- Process control errors
- Misreported lab results
- Health and safety risks
For example:
- Air pollutant concentrations are usually expressed as ppm by volume.
- Water contaminants are typically measured as ppm by mass (mg/kg or mg/L).
Understanding when to use each form — and how density affects interpretation — is essential for accurate concentration measurement.
This comprehensive guide explains the difference, provides calculation walkthroughs, case studies, industry examples, comparison tables, and practical troubleshooting insights.
What Is PPM? A Brief Refresher
Parts per million (ppm) represents one part of a substance per one million parts of a mixture.
General ppm formula:
PPM = (Amount of solute / Amount of mixture) × 1,000,000
However, the “amount” can be measured in:
- Mass (grams, kilograms)
- Volume (liters, cubic meters)
This is where the distinction begins.
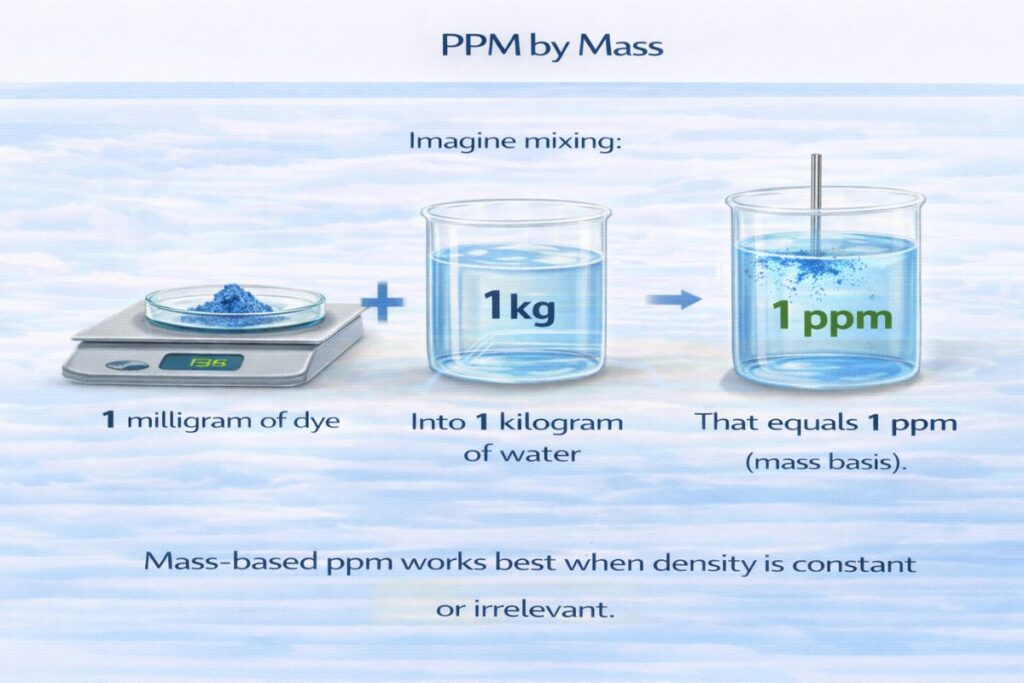
Understanding PPM by Mass
What Is PPM by Mass?
PPM by mass expresses the mass of a solute per mass of the total mixture.
Formula:
PPM (mass basis) = (Mass of solute / Mass of mixture) × 1,000,000
Common Units
- mg/kg
- g/ton
- µg/g
For water:
1 ppm ≈ 1 mg/L
(because 1 liter of water ≈ 1 kg)
Where PPM by Mass Is Used
1. Water Quality Testing
Heavy metals: Lead, arsenic, mercury
Example: Lead limit = 0.015 ppm
2. Soil Analysis
Nutrient concentration
Example: Nitrogen = 25 ppm
3. Industrial Product Specifications
Impurities in polymers or fuels
Example: Sulfur < 10 ppm
alculation Walkthrough: PPM by Mass
Scenario
A 2,000 kg batch contains 4 grams of impurity.
Step 1: Convert grams to kilograms
4 g = 0.004 kg
Step 2: Apply formula
PPM = (0.004 / 2000) × 1,000,000
= 2 ppm
This is a typical ppm example in manufacturing.
Understanding PPM by Volume
What Is PPM by Volume?
PPM by volume expresses the volume of a gas per volume of air (or another gas mixture).
Formula:
PPM (volume basis) = (Volume of gas / Volume of mixture) × 1,000,000
Often written as:
ppm(v/v)Where PPM by Volume Is Used
1. Air Quality Monitoring
Carbon monoxide (CO) = 9 ppm (8-hour limit)
2. Industrial Gas Mixtures
Calibration gases
3. HVAC and Indoor Air Monitoring
CO₂ levels measured in ppm(v/v)
Calculation Walkthrough: PPM by Volume
Scenario
A lab injects 0.5 mL of gas into 500 liters of air.
Convert liters to mL:
500 L = 500,000 mL
Apply formula:
PPM = (0.5 / 500,000) × 1,000,000
= 1 ppm (v/v)
This is common in environmental monitoring.

The Critical Role of Density
Why Density Changes Interpretation
Mass and volume are connected through density:
Density = Mass / Volume
For liquids other than water, 1 ppm ≠ 1 mg/L.
Example: Density Impact
Liquid density = 1.2 kg/L
Measured concentration = 10 mg/L
Convert to mass-based ppm:
PPM = 10 / 1.2
= 8.33 ppm
Ignoring density causes a 20% error.
Comparison Table: PPM by Volume vs PPM by Mass
| Feature | PPM by Mass | PPM by Volume |
|---|---|---|
| Basis | Mass ratio | Volume ratio |
| Typical Media | Liquids, solids | Gases |
| Units | mg/kg, mg/L | ppm(v/v) |
| Density Impact | Yes | Yes (indirect) |
| Common Use | Water testing | Air quality |
| Conversion Needed? | Sometimes | Often |
Converting Between PPM by Volume and Mass
Conversion requires:
- Molecular weight
- Temperature
- Pressure
- Gas constant
Formula for Gas Conversion
At 25°C and 1 atm:
mg/m³ = (ppm × Molecular Weight) / 24.45Calculation Example
CO molecular weight = 28 g/mol
Measured concentration = 50 ppm (v/v)
mg/m³ = (50 × 28) / 24.45
= 57.27 mg/m³
This conversion is essential for regulatory compliance.
Industry Case Studies
Case Study 1: Air Pollution Misinterpretation
A factory reported:
CO concentration = 35 ppm
Regulator required mg/m³ reporting.
Incorrect conversion caused underreporting by 12%.
Result: Fine and compliance review.
Case Study 2: Chemical Reactor Contamination
Process engineer misinterpreted ppm(v/v) as ppm(mass).
Impurity was 5 ppm by volume, not mass.
Batch failure cost $0.20 Million
Lesson: Context matters.
Environmental vs Industrial Interpretation
| Context | Interpretation |
|---|---|
| Environmental Air | ppm(v/v) |
| Drinking Water | ppm(mass) |
| Soil Testing | ppm(mass) |
| Gas Calibration | ppm(v/v) |
| Petrochemical Streams | Both |
Understanding context prevents costly errors.
Long-Tail Keyword Focus — When to Use PPM by Volume vs PPM by Mass
Use ppm by volume for gases, especially in:
- Indoor air monitoring
- Emission reporting
- Atmospheric research
Use ppm by mass for liquids and solids, including:
- Water quality analysis
- Soil testing
- Food safety
- Industrial materials
Common Mistakes in PPM Interpretation
1. Ignoring Density in Liquids
2. Confusing mg/L with ppm in Non-Water Liquids
3. Using Gas ppm Without Temperature Correction
4. Reporting Without Specifying Basis
Always label:
- ppm(w/w)
- ppm(v/v)
- ppm(w/v)
Tools & Calculators for Accurate Conversion
Manual conversions increase risk.
Trusted Platform: ppmcalculation.com
ppmcalculation.com offers:
Benefits
- Accurate ppm formula implementation
- Density-aware calculations
- Fast and mobile-friendly
- No registration required
Using digital tools reduces misinterpretation
FAQs: PPM by Volume vs PPM by Mass
1. Is ppm always mg/L?
Only for water-based solutions with density ≈1 kg/L.
2. Why is air measured in ppm by volume?
Because gases mix proportionally by volume under similar conditions.
3. Can ppm(v/v) equal ppm(w/w)?
Only if density equals 1 and molecular weights align — rare.
4. Does temperature affect ppm by volume?
Yes, gas volume changes with temperature and pressure.
5. Which is more accurate?
Both are accurate when used in correct context.
6. How do regulators specify ppm?
They clearly state basis (mass or volume).
Mastering PPM by Volume vs PPM by Mass
Understanding PPM by Volume vs PPM by Mass is essential for accurate scientific reporting and regulatory compliance.
Key Takeaways
✔ PPM by mass applies to liquids and solids
✔ PPM by volume applies to gases
✔ Density affects interpretation
✔ Temperature influences gas ppm
✔ Proper conversion prevents errors
✔ Digital tools enhance reliability
The difference is not mathematical complexity—it is contextual accuracy.
Related PPM Calculators
Explore more water quality and chemistry tools: