Healthy soil is the foundation of productive agriculture, sustainable landscaping, and environmental protection. However, soil quality cannot be judged by appearance alone. Hidden within every handful of soil are nutrients, salts, metals, and contaminants that directly affect plant growth and ecosystem health.
This is where measuring ppm in soil becomes essential.
Using parts per million (ppm), scientists, agronomists, environmental engineers, and farmers quantify nutrient levels, detect pollution, and optimize fertilizer use. Incorrect ppm in soil measurements can lead to:
- Over-fertilization and soil degradation
- Crop nutrient deficiencies
- Heavy metal contamination
- Reduced yields and profitability
In this comprehensive guide, you will learn professional techniques for soil sampling, laboratory preparation, ppm calculation, interpretation, and practical use—bridging the gap between technical science and everyday application.
What Does PPM Mean in Soil Analysis?
Understanding Parts Per Million in Soil
In soil science, parts per million (ppm) represents the mass of a substance per million parts of dry soil.
1 ppm = 1 mg/kg (in dry soil)
This equivalence is widely used in soil testing laboratories.
Why PPM Is Used in Soil Testing
PPM is preferred because:
✔ Soil nutrients exist in trace amounts
✔ Regulatory limits are expressed in ppm
✔ Instruments report in ppm or mg/kg
✔ Easy comparison across regions
It provides a standardized approach to concentration measurement.
Importance of Measuring PPM in Soil
1. Nutrient Management
Soil ppm values guide fertilizer application.
Example:
| Nutrient | Optimal Range (ppm) |
|---|---|
| Nitrogen (N) | 20–50 |
| Phosphorus (P) | 15–40 |
| Potassium (K) | 120–250 |
| Zinc (Zn) | 1–5 |
2. Environmental Protection
Monitoring heavy metals prevents contamination.
- Lead: <50 ppm
- Cadmium: <3 ppm
- Mercury: <1 ppm
3. Agricultural Productivity
Balanced nutrient ppm improves:
- Root development
- Water retention
- Crop resistance
4. Land Remediation
Soil cleanup projects depend on accurate ppm data.
Common Soil Parameters Measured in PPM
| Parameter | Role | Typical Range |
|---|---|---|
| Nitrogen | Growth | 20–50 ppm |
| Phosphorus | Roots | 15–40 ppm |
| Potassium | Yield | 120–250 ppm |
| Iron | Chlorophyll | 10–50 ppm |
| Lead | Toxicity | <50 ppm |
| Salinity | Osmotic stress | <2,000 ppm |
These parameters determine soil suitability.
Step 1: Proper Soil Sampling Techniques
Accurate measurement begins with proper sampling.
Field Sampling Guidelines
✔ Use clean stainless steel tools
✔ Sample 10–15 locations per field
✔ Avoid fertilizer bands
✔ Remove surface debris
✔ Sample at consistent depth (15–20 cm)
Composite Sampling Method
Combine multiple subsamples into one representative sample.
Visual Explanation: Soil Sampling
Imagine a grid over your field. At each intersection, collect a small soil core and mix them into a composite sample. This reduces local variability.
Step 2: Soil Sample Preparation in the Laboratory
Drying
Air-dry samples at 25–30°C.
Purpose:
- Prevent microbial activity
- Standardize moisture
Grinding and Sieving
- Grind to <2 mm
- Remove stones and roots
Homogenization
Mix thoroughly to ensure uniform composition.
Step 3: Chemical Extraction for PPM Measurement
Nutrients must be extracted into solution.
Common Extraction Methods
| Method | Application |
|---|---|
| Bray-1 | Phosphorus |
| Olsen | Alkaline soils |
| Mehlich-3 | Multi-nutrient |
| DTPA | Micronutrients |
Example: Mehlich-3 Extraction
- Weigh 2.5 g soil
- Add 25 mL extractant
- Shake for 5 min
- Filter solution
Result: Extract ready for analysis.
Step 4: Instrumental Analysis Techniques
1. Atomic Absorption Spectroscopy (AAS)
- Metals detection
- High accuracy
2. ICP-OES / ICP-MS
- Multi-element analysis
- ppb–ppm sensitivity
3. UV-Visible Spectrophotometry
- Nutrients (P, N)
- Cost-effective
4. Portable Soil Sensors
- Field testing
- Rapid screening
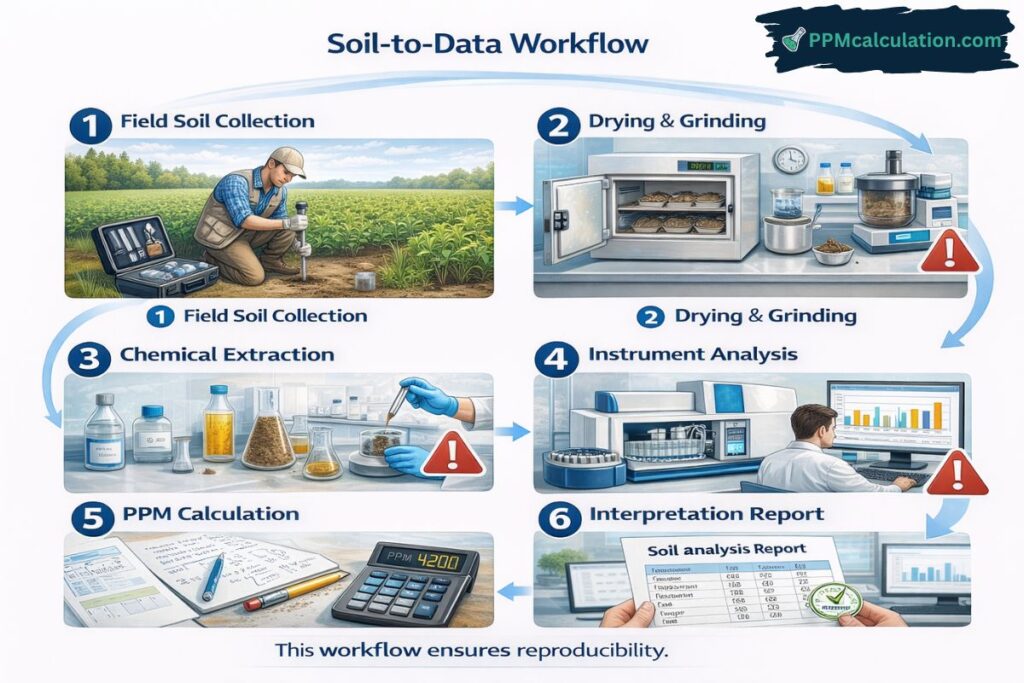
Step 5: PPM Formula for Soil Analysis
Standard PPM Formula
PPM = (Mass of Analyte / Mass of Soil) × 1,000,000
Laboratory-Based Formula
When extraction is involved:
PPM = (C × V × D) / W
Where:
- C = Instrument reading (mg/L)
- V = Extract volume (L)
- D = Dilution factor
- W = Soil weight (kg)
Calculation Walkthrough: Phosphorus in Soil
Given Data
- Instrument reading: 4 mg/L
- Extract volume: 0.025 L
- Soil weight: 0.0025 kg
- Dilution: 1
Step 1: Apply Formula
PPM = (4 × 0.025 × 1) / 0.0025
= 40 ppm
Interpretation
Phosphorus = 40 ppm (Upper optimal range)
This is a practical ppm example used in soil labs.
PPM to mg/L Conversion in Soil Testing
When Is It Needed?
Instrument readings are often in mg/L, while soil results require ppm.
Conversion Principle
In extracts:
mg/L ≠ ppm (directly)
Must use extraction volume and soil mass.
Example
Reading = 2 mg/L
Volume = 50 mL
Soil = 5 g
PPM = (2 × 0.05) / 0.005 = 20 ppm
Comparison: Laboratory vs Field PPM Testing
| Feature | Laboratory | Field Kits |
|---|---|---|
| Accuracy | High | Moderate |
| Cost | Medium–High | Low |
| Speed | Slow | Fast |
| Elements | Multiple | Limited |
| Compliance | Suitable | Screening |
Professional decisions require lab data.
Industry Case Study 1: Fertilizer Optimization in Wheat Farming
Background
A wheat farm experienced declining yields.
Soil Test Results:
- Nitrogen: 12 ppm
- Recommended: 25 ppm
Action
Adjusted fertilizer based on ppm data.
Outcome
- Yield increase: 28%
- Cost reduction: 15%
- Improved soil health
Industry Case Study 2: Heavy Metal Assessment Near Industrial Site
Scenario
Land near battery factory tested for contamination.
Results:
- Lead: 180 ppm
- Safe limit: 50 ppm
Impact
- Land use restrictions
- Remediation initiated
- Soil replacement
Accurate ppm measurement enabled legal compliance.
Interpreting Soil PPM Results
Nutrient Classification
| Range | Status |
|---|---|
| Low | Deficient |
| Medium | Adequate |
| High | Excessive |
| Very High | Toxic |
Example Interpretation
Potassium = 300 ppm
Status: Excessive
Action: Reduce fertilizer
Tools & Calculators for Soil PPM Analysis
Manual calculations are prone to errors. Digital tools simplify analysis.
Trusted Platform: ppmcalculation.com
ppmcalculation.com provides reliable online tools for soil analysts and agronomists:
Advantages
- Laboratory-grade formulas
- Instant conversion
- Mobile compatibility
- No sign-up required
- Error reduction
These tools enhance accuracy in soil testing workflows.
Common Mistakes in Measuring PPM in Soil
1. Poor Sampling
Non-representative samples distort results.
2. Moisture Neglect
Wet soil skews ppm values.
3. Cross-Contamination
Dirty tools introduce metals.
4. Incorrect Dilution Factors
Calculation errors inflate values.
5. Misinterpretation
Ignoring soil type and crop needs.
Frequently Asked Questions (FAQs)
1. Is ppm in soil the same as mg/kg?
Yes. For dry soil, 1 ppm = 1 mg/kg.
2. How often should soil ppm be tested?
At least once per growing season, ideally before planting.
3. Can farmers measure ppm at home?
Basic kits exist, but laboratory testing is more reliable.
4. Why are different extraction methods used?
Because soil chemistry varies with pH, mineralogy, and texture.
5. What is a dangerous lead level in soil?
Above 50 ppm is considered unsafe for agriculture.
6. Can ppm values change seasonally?
Yes. Rainfall, fertilization, and crop uptake affect ppm.
Mastering the Science of Measuring PPM in Soil
Accurate measuring ppm in soil is essential for sustainable agriculture, environmental safety, and land management.
Key Takeaways
✔ PPM reflects nutrient and contaminant levels
✔ Proper sampling is critical
✔ Extraction and analysis ensure accuracy
✔ Correct ppm calculation prevents mismanagement
✔ Digital tools improve reliability
From fertilizer planning to pollution control, understanding ppm in soil empowers data-driven decisions.
Related PPM Calculators
Explore more water quality and chemistry tools: